Confinement of bioactive secondary metabolites from callus culture of Phyllanthus amarus L. and evaluation of its microbial activity
Avinash Singh*, Anam Taufeeq, Samra Fatima
Department of Biotechnology, Axis Institute of Higher Education, Kanpur, Uttar Pradesh, India
Abstract
Phyllanthus amarus is one of the very important medicinal plants. Secondary metabolites originating from plants are playing significant role in medical field. Thus, this study was aimed to extract secondary metabolites from callus induced from leaf tissues of P. amarus of the different growth regulators [ 2,4-dichlorophenoxy acetic acid (2,4-D), 6-Benzyl aminopurine (BAP)] tested for callus induction, the leaf segment inoculated in MS media containing 1mg/l BAP and 1mg/l 2,4-D showed profuse and consistent callusing. The callus produced were dried at 25±2 OC overnight and subjected for metabolite extraction. About 2.3gm dried compounds was extracted from 10.0 gm of dried callus. The methanolic solution (2 mg/ml) of dried compounds tested for the presence of phenolics, flavonoids and antioxidant activity showed 0.023mg GAE/g total phenolics, 0.19 mg QE total flavonoids and 93.51 percent activity, respectively. Of the four bacteria (Escherichia coli, Morgan morganii, Escherichia fecalis and Staphylococcus aureus) tested for antimicrobial activity, the E. coli and M. morganii were found sensitive to the methanolic extract (1 mg/ml) of callus. Thus, this study showed that the callus culture of P. amarus is source of various bioactive compounds and could be exploited for commercialization of various bioactive compounds.
Keywords: Phyllanthus amarus; Callus; leaf section; antimicrobial activity; 2,4-dichlorophenoxy acetic acid
Corresponding Author
Avinash Singh Email: dr.avinashsingh99@gmail.com
-
- Introduction
The annual plant Phyllanthus amarus, a member of the Phyllanthaceae family, ranges in height from 10 to 60 cm and has a single main stem that may branch (de Oliveira et al., 2020). Since ancient times, India, China, and South America have employed a variety of plants of the genus Phyllanthus (common name: leaf flower) (Euphorbiaceae), used in the diagnosis of urinary tract ailments and liver (Sparzak-Stefanowska et al., 2022). In recent time, pharmacological research actively focus on many leaf flower species because of their hepatoprotective or antiviral activity averse to hepatitis B virus (HBV), cytotoxic, anti-inflammatory, and antibacterial activity. Up to an elevation of 800-1000 m MSL, the annual herb Phyllanthus amarus grows natively in tropical and subtropical areas of Central and South Asia (Sparzak et al., 2015). For the treatment of ailments of the liver, genitourinary system, kidney, and stomach, the plant is important in the Ayurvedic medical system. Antihepatotoxic, antilytic, anti-human immunodeficiency virus (HIV), anti-inflammatory, antihypertensive, and anti-hepatitis B properties are some of its clinical effects (Karthik et al., 2019) Conventional Phyllanthus species farming is generally done for therapeutic reasons takes place in greenhouse or in the natural environment but is obstructed by the shallow formation of desired secondary metabolites such as lignans, along with the seeds’ inherent dormancy, which restricts its dissemination for large-scale farming (Almeida et al., 2011). According to Gandhi et al. (2015) plant tissue culture procedures may make it possible to construct in vitro cultivation that accumulated intriguing secondary metabolites unescorted by the drawbacks of field farming and to manipulate plant development in a more consistent, homogenous, and accurate manner.
Callus culture in vitro has been acknowledged as substitute for generating biomass intended to produce metabolites, particularly for the simplicity of initiation and dissemination. This is easy and simple procedure than organogenic or embryogenic responses and it elevates the production of in vitro plant biomass, one of the main economic constraints in the secondary metabolite formation in vitro (Cardoso et al., 2019). The generation and multiplication of calli in vitro also enable good reproducibility and standardisation of result (Keshvari et al., 2018). Miroshnichenko et al. (2017) mounted that plant development regulators (PDRs) are inclined in the baptism and expansion of calli, whilst auxins and cytokinins oversee cell division and adaptation in tissues cultured in vitro. Among the most popular agents used for in vitro callus development is auxin 2,4-dichlorophenoxyacetic acid (2,4-D), which is incredibly beneficial for the induction and proliferation of these calli. When combined with 6-benzyladenine, its effectiveness can be strengthened (BA). Blending in vitro calli used for cell mass proliferation with chemical inducers that excite secondary metabolite trail can result in a sophisticated method for biosynthesis that enhances the yield of secondary metabolites under in vitro conditions (Schmedes. 2020). Salicylic acid along with chitosan have been used as inducers in numerous research on medicinal plants with promising results, activating various metabolic pathways, and increasing the content of certain phenolic compounds in plants (El-Beltagi et al., 2022).
Additionally, calli may be a reservoir of novel pharmaceutical chemicals that are rarely found in typically differentiated tissues like the stems, roots and leaves of medicinal plants grown in the field. Thus, the manufacture of plant-derived pharmaceutical chemicals from appropriate medicinal plants, such as Phyllanthus species, can be effectively carried out in a bioreactor by callus cell growth (de Oliveira et al., 2020). In this regard, the chief intention of the study is to isolate bioactive secondary metabolite from Phyllanthus amarus and evaluation of its antimicrobial activity against Escherichia coli, Escherichia fecalis and Staphylococcus aureus.
2. Materials & Methods
2.1 Plant material and Culture Initiation: The explants (small shoots, Fig. 1A) were collected from the plants growing in campus of Axis Institute of Higher Education, Kanpur Uttar Pradesh, India. To establish callus cultures the leaves from the collected shoots were cleansed under water from a piped supply, then treated in mixture of 1% (v/v) cetrimide and 2% (v/v) sodium hypochlorite solution for 10 min, chased by rinsing thoroughly with sterile distilled water (DW). The leaves were then rinsed by 70% ethanol for 30 seconds and washed with sterile (DW) before surface sterilization, using 0.05% (w/v) mercuric chloride (HgCl2) solution for 3-4 min inside the laminar-air-flow cabinet. After three washing with sterile DW, the leaves were cut into small sections (about 1.0 cm2) and were cultured for callus induction on Murashige and Skoog (MS) medium having 3% (w/v) sucrose and supplemented with either different concentrations (0.0, 0.1, 1.0 and 5.0 mg/l) of 2,4-dichlorophenoxy acetic acid (2,4-D) and 6-benzyl-aminopurine (0.1 and 1.0 mg/l BAP) alone or in mixture of 1.0 mg/l 2,4-D with 0.1 or 1.0 mg/l BAP. Agar at 0.8% (w/v) was used to solidify the media. 1.0 mg/l 2,4-D and 1.0 mg/l BAP were added to fresh agar gelled MS medium with the callus initiated after 30 days to promote proliferation.
In each experiment, the medium’s pH was adjusted to 5.8 0.02 before autoclaving for 15 minutes at 1.06 kg/cm2 and 121 °C. Culture were incubated at 25 ± 2ºC and under 16 hours photo period with intensity 1000-2000 lux, provided with cool white fluorescent tube-lights. There are 12 replicates of each treatment, and the experiment was run at least three times. The information was gathered in terms of the proportion of responsive explants to the total number of leaf sections cultivated. The lowest significant difference (LSD) test was used to equate the mean values of the various treatment groups. After monitoring the fresh weight of the callus, which was picked between 5 and 6 weeks into the culturing process for metabolite extraction, they were dried in a hot air oven at 25±2 OC until a steady weight was attained. To avoid the thermal degradation of metabolites, the drying temperature was kept low. Methanol was used as a solvent in a Soxhlet extractor to extract the metabolites from the dried callus. The resulting extract was then dried, weighed, and kept at room temperature for later use.
2.2 Identification of the phenolic content: The Folin-Ciocalteu reagent was used to determine the total phenolic component in the plant extract (Javanmardi et al., 2003). The reagent oxidised the phenolic molecule to phenolates at an alkaline pH in a saturated sodium carbonate solution, producing a blue molybdenum-tungsten complex that could be seen at 764 nm. A plant extract volume of 50 l was combined with 2.5 ml of diluted Folin-reagent Ciocalteu’s and 2.0 ml of sodium carbonate (7.5%, w/v), along with 1 ml of methanol and 2 mg of the dried chemical. The sample was then left to develop the blue colour for 15 minutes at 45°C. By comparing to a reference gallic acid, the total phenolic content was determined and reported as milligrams of gallic acid equivalents per gramme of extract (mg GAE/g extract).
2.3 Determining the content of flavonoid: The aluminium chloride colorimetric method was used to quantify the amount of total flavonoid present (Joubert et al., 2008). 1.5 ml of methanol, 0.1 ml of 10% aluminium chloride, 0.1 ml of (1 mol/l) potassium acetate, and 2.8 ml of distilled water were combined with the leaf extracts (0.5 ml of 1:10 g/ml in methanol). The reaction mixture’s absorbance was assessed at 415 nm after 30 min of room temperature incubation. The standard curve was created by making methanol solutions of different quercetin concentrations. Quercetin equivalent was used to express the outcome (mg QE/g extract).
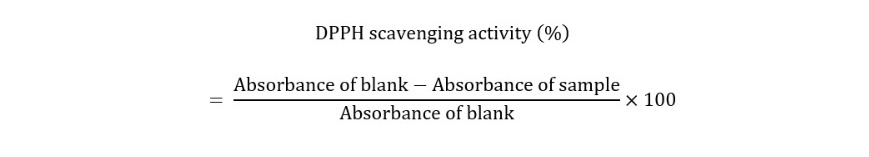
2.4 DPPH radical scavenging assay: The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical was used to test the plant extracts’ ability to scavenge free radicals (Thitilerdecha et al., 2010). 2.95 ml of DPPH solution was combined with about 50 l of plant extract (6.4 mg of DPPH in 100 ml of absolute ethanol). After the solution had been allowed to stand at room temperature for 30 minutes in the dark, the absorbance at 515 nm was measured. The control solution was ethanol. The scavenging activity of the DPPH radicals was calculated by plant extracts according to the following formula
2.5 Antimicrobial assay: The spectrum of Antibacterial activity was studied using 4 different species of bacteria (Escherichia coli, Morgan morganii, Escherichia fecalis and Staphylococcus aureus). In vitro antibacterial assay was carried out by Disc diffusion technique. Filter paper discs were impregnated with known amount of test sample (2mg dried compound dissolved in 1ml of methanol) of Phyllanthus amarus. The discs were then placed individually using a sterile forceps on culture plate inoculated (spread) with test bacterial cultures and incubated at 37OC for overnight. After overnight incubation, plates were observed and data for zone of inhibition were recorded.
3. Results & Discussion
Callus formation was observed from in vitro cultured leaf tissues. The medium devoid of growth regulator did not show any response and the cultured leaf tissues died with 2 weeks of culture initiation. Of the various treatments of 2,4-D and BAP alone, callus induction was recorded on medium having 2,4-D. All the concentration of 2,4-D tried were effective in callus initiation but the percentage of responsive cultures as well as amount of callus produced were varied depending on the concentration of 2,4-D used in the medium. The initiation of callus was observed within two weeks of culture initiation on the medium supplemented with 1 am 5 mg/l 2,4-D and of these two concentrations of 2,4-D the percent response for callus initiation was high on medium having 1 mg/l 2,4-D (Table 1). The addition of 2,4-D for callus induction from leaf tissues has been also reported in Sapindustri foliates (Singh et al., 2015). On BAP containing medium though the cultured leaf sections showed swelling and get increased from initial size but no callus induction was recorded. In this study maximum response (66.67) for callus induction was recorded on medium with 1.0 mg/l 2,4-D and 1.0 mg/l BAP. The callus obtained was brownish and slightly compact. Manipulations of Auxins/Cytokinins ratio to achieve better induction of callus and similar observation have also been reported in other medicinal plants (Yang et al., 2010). The consistent callus growth was observed after each subculture on MS medium having 1mg/l BAP and 1mg/1 2,4-Dichlorophenoxyacetic acid (Table 2; Fig. 1B & C).
The amount of extract obtained after methanolic extraction is depicted in Fig 2. About 2.30 gm dried extract was extracted from 10.21 gm dried callus. The methanolic extract of E. alba contained phenolic (0.023mg GAE/g) and flavonoids (0.019 mgQE) compounds even the free radical scavenging activity (Table 3). This investigation showed that the extract contains phenolic, flavonoids and antioxidant activity. Numerous conditions, including atherosclerosis, angina pectoris, neurological illnesses, and cancer, are influenced by free radicals produced by oxidative stress. Due to their scavenging function, antioxidants may be helpful in the treatment of certain disorders (Uddin et al., 2010). A sensitive approach to assess the antioxidant activity of plant extracts is the DPPH free radical stability technique (Jasprica et al., 2007).
The bacteria E. coli and M. morgani were found sensitive to methanolic extracts of Phyllanthus amarus (1mg/ml). E. fecalis and S. aureus showed resistance against to above extract. Of the E. coli and M. morgani, the E. coli was more sensitive to the extract (Table 4). The result of antibacterial assay confirms that Methanolic extract have great potential to kill pathogens like E. coli and M. morganii. Similar results of antimicrobial activity of aerial parts crude extracts of Phyllanthus amarus reported earlier (Uddin et al., 2010; Bakht et al., 2011). Thus, the experimental findings of the present investigation revealed that the dedifferentiated cells of leaf explants could be used for production of various bioactive compounds of medicinal value. Further identification and scaling up of these compounds by cultured cells are needed to reduce or eliminate the need to cultivate the source plant under variable climatic conditions.

Table 1: Effect of growth regulator (GR) on callus induction from leaf tissues
| Concentration of GR (mg/l) | Percent response | Callus amount | |
| 2,4-D | BAP | ||
| 0.0 | 0.0 | 0.0 | – |
| 0.0 | 0.1 | 0.0 | – |
| 0.0 | 1.0 | 0.0 | – |
| 0.1 | 0.0 | 13.89a | + |
| 1.0 | 0.0 | 58.32b | ++ |
| 5.0 | 0.0 | 41.65c | ++ |
| 1.0 | 1.0 | 66.67b | +++ |
Values followed by different letters are significant (P< 0.05)- no callusing; + minor; ++ medium; +++ profuse callusing
Table 2: Callus growth during subculture
| Subculture Number (At 30 days interval) | Callus weight (On day of subculture) (In gm) | Callus weight (After 30 days) (in gm) (Mean± S.D) |
| 1st Subculture | 0.35 ± 0.07 | 2.51 ± 0.07 |
| 2nd subculture | 0.37± 0.03 | 2.30 ± 0.91 |
| 3rd subculture | 0.32± 0.03 | 2.21 ± 0.76 |
Table 3: Polyphenolic compound and antioxidant activity of methanolic extract from callus of Phyllanthus amarus.
| Methanolic extract | Content (Mean ± SD) |
| Phenols (mg GE) | 0.023 ± 0.01 |
| Flavonoids (mg QE) | 0.019 ± 0.007 |
| DPPH (% activity) | 3.94 |
Table 4: Effect of antimicrobial activity of methanolic extract on some bacterial strains.
| Bacteria | Inhibition zone (in mm) (Mean ± SD) |
| Escherichia coli | 5 ± 1.41 |
| Morgan morgani | 3 ± 1.41 |
| Escherichia fecalis | 0.0 |
| Staphylococcus aureus | 0.0 |
4. Conclusion
Our investigation provided the construction of an efficient procedure for the expansion of calli of P. amarus utilisingMS medium having 1mg/l BAP and 1mg/l 2,4-D-dichlorophenoxyacetic acid. The amount of phenolics, flavonoids, and antioxidant activity in the dried components’ methanolic solution (2 mg/ml) was 0.023 mg GAE/g total phenolics, 0.19 mg QE total flavonoids, and 93.51 percent activity, respectively. When the antibiotic activity of the four bacteria (Escherichia coli, Morgan morganii, Escherichia fecalis, and Staphylococcus aureus) was evaluated, only E. coli and M. morganii were shown to be responsive to the callus’ methanolic extract (1 mg/ml). As a result, our work demonstrated that P. amarus callus culture is a source of a variety of bioactive compounds and might be used to commercialise a variety of bioactive substances.
Acknowledgement
I would like to mention thanks to my supervisors Dr. Madhulika Singh and Dr. Ajay Kumar, for their immense support throughout the work. I also want to appreciate the technical assistant of biotechnology laboratory Chhatrapati Shahu Ji Maharaj University for helping me in the project.
My co-workers Ms. Anam Taufeeq and Ms. Samra Fatima who helped me with data collecting and analysis, deserve special gratitude. I want to show gratitude towards Axis Colleges, Kanpur for their immense support in the study. I would like to mention thanks to my parents for their never-ending help with my studies.
Author’s Contribution
Avinash Singh was responsible for the tests’ conception, planning, execution, and analysis. He also prepared the text and created the tables and figures. The manuscript was edited and updated by Anam Taufeeq. The manuscript was formatted by Samra Fatima in accordance with the journal’s guidelines.
Declaration of Interest
There is no competing interest between the authors
Ethical Statement
No animal of human studies has been conducted
Funding Statement
No funding was received
References
Bakht, J., Islam, A., Ali, H., Tayyab, M., & Shafi, M. (2011). Antimicrobial potentials of Eclipta alba by disc diffusion method. African Journal of Biotechnology, 10(39), 7658–7667.
Schmedes, P. S. (2020). Investigating hatchery and cultivation methods for improved cultivation of Palmaria palmata (Doctoral dissertation, Ph. D. dissertation).
Uddin, M. N., Rahman, M. A., Ahmed, N. U., Rana, M. S., Akter, R., & Chowdhury, A. M. A. (2010). Antioxidant, cytotoxic and antimicrobial properties of Eclipta alba ethanol extract. International Journal of Biological and Medical Research, 1(4), 341–346.